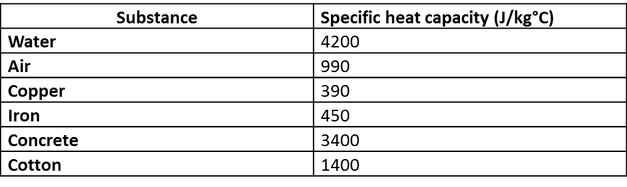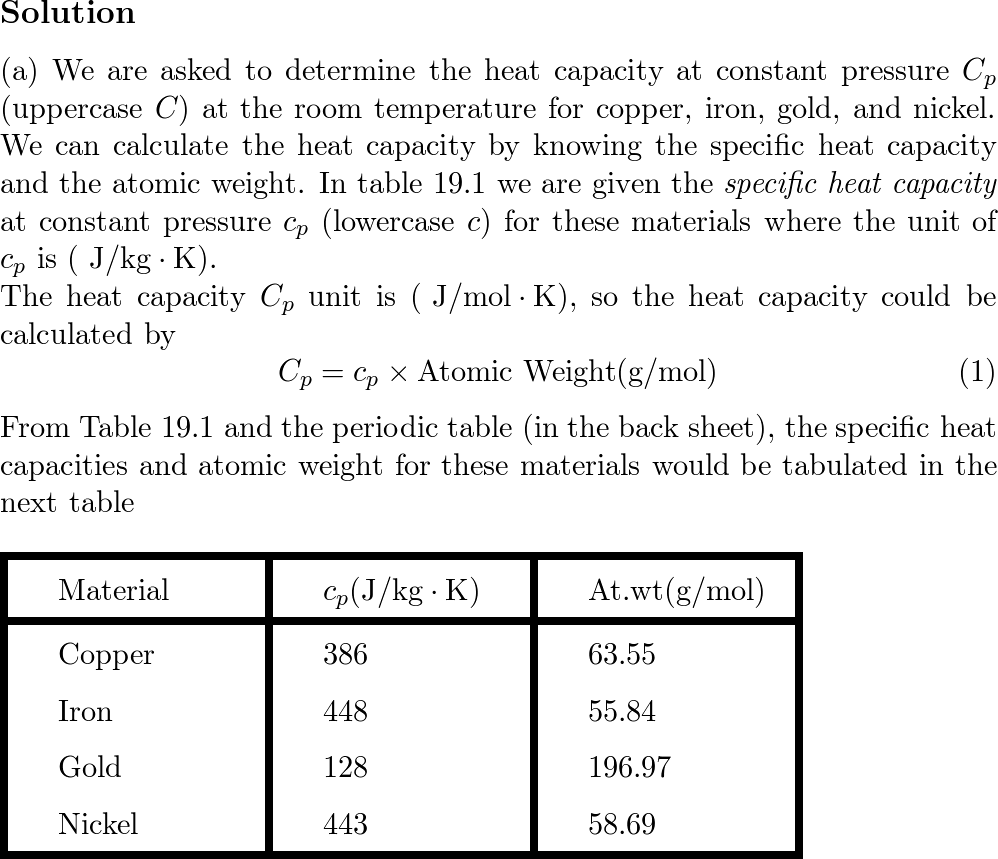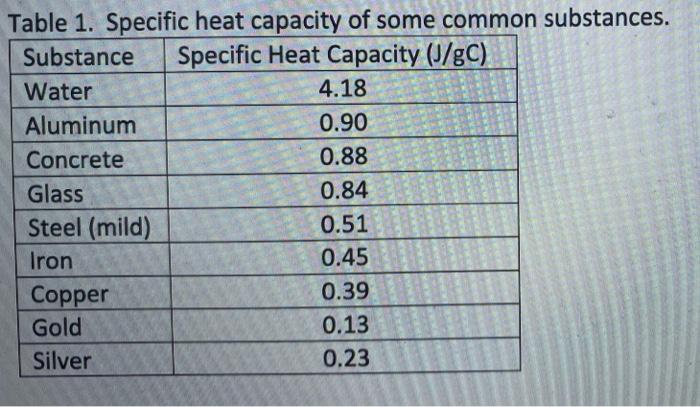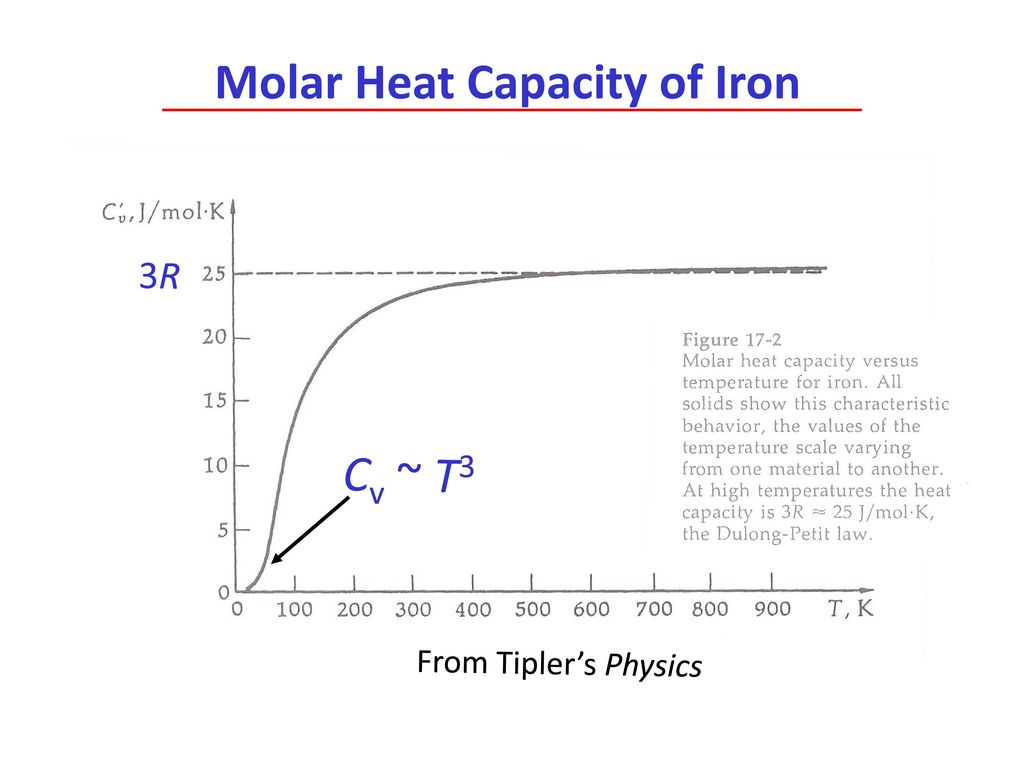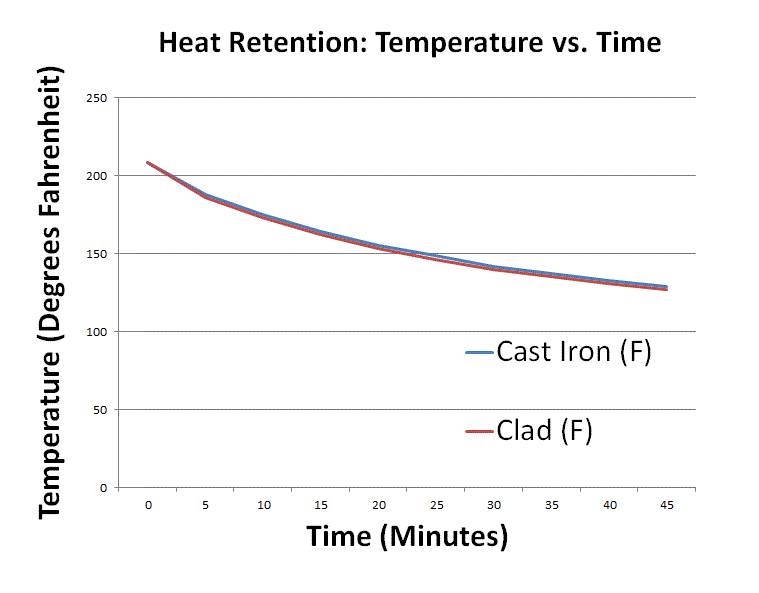
Heat retention myths and facts: Does cast iron hold heat better than clad? Is clad better than disc-base at retaining heat longer?

Color online) Temperature-dependent specific heat capacities of (a)... | Download Scientific Diagram

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram

Calculate the amount of heat required to raise the temperature of 5 g of iron from `25^(@)C \"to\" - YouTube

The Heat Capacity and Thermodynamic Properties of the Iron Oxides and Their Relation to the Mineral Core of the Iron Storage Protein Ferritin | Semantic Scholar

The formation of Fe_2O_3 from iron and oxygen at 298 K is depicted below. 2Fe(s)+\frac{3}{2} O_2(g) \rightarrow Fe_2O_3(s) Assuming the heat capacities do not change with temperature, what would the | Homework.Study.com
Molten iron is extremely hot, averaging about 1,500 C. The specific heat of iron is 0.46 J/gC. How much heat is released to the atmosphere when 1 kg molten iron cools to







![Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram Molar heat capacity of iron. Symbols indicate measurements from [10]. | Download Scientific Diagram](https://www.researchgate.net/publication/332219094/figure/fig1/AS:744154090987520@1554431569138/Molar-heat-capacity-of-iron-Symbols-indicate-measurements-from-10.png)